Introduction
Carcinoma of the prostate (CaP) is the most common malignancy
in American males, with 17.1% lifetime probability of developing this
disease (1). Management of patients with CaP in the United States
is a success story in oncology. Over the past 25 years, there has been
a sharp increase in the 5−year survival rates. It was 69% in 1977 and
it increased to 100% in 2002 (Table 1) (1). This improvement was
driven by multiple factors, which included our better understanding
of the disease process and its risk factors, better ability to define anatomic
extent of the tumor, an ability to diagnose the majority (91%)
of patients with localized tumors at presentation and a major evolution
of surgical and radiotherapy techniques. Additionally, there has
been generally a well−integrated multidisciplinary approach to the management
of CaP with an active participation of urologists, radiation
oncologists, radiologists, pathologists and appropriately trained
nursing staff. All of the above facilitated the improvement of the
patient survival and the quality of life.
|
|
 |
Radiotherapy
Radiotherapy (RT) is a well−established and widely accepted curative
treatment modality for selected patients with localized (CaP). RT
treatment outcomes have substantially improved over the past two
decades and became similar to those obtained with contemporary
radical prostatectomy (RP) (2−7). Contemporary RT utilizes 3−D
conformal or intensity modulated radiation therapy (IMRT). RT
treatment planning and delivery represent a complex process which
extensively utilizes imaging and computers as well as highly trained
radiation oncologists, technical and nursing staff. Accuracy of treatment
planning and delivery is of critical importance as the treatment
outcome depends on it. Radiation dose and schedules are designed
based on patient risk factors. Generally, patients with favorable
prognosis receive at least 70 Gy and those in intermediate and unfavorable
risks groups receive up to 81Gy. RT is given at 1.8−2.0 Gy
daily over a period of 7 to over 8 weeks. Patient quality of life during
the treatment course is reasonably good with a minor toxicity common
(40%) but easy to control and major toxicity rare (<3%).
|
5-Year overall survival USA 1996-2002 (1) |
 |
In a report from Memorial Sloan−Kettering Cancer Center in New York
on IMRT treated patients with localized CaP, the 8−year actuarial
PSA relapse free survival for the favorable, intermediate and unfavorable
groups was 85, 76 and 72%, respectively (2). The 8−year
cause specific survival for the above risk groups was 100%, 96% and
84%, respectively. This report is representative of other published
studies from large American university centers.
Biochemical relapse
Unfortunately, the above excellent 5 or 8−year overall and disease
free survival does not address well the existence of late (10 years)
PSA relapse, which has been well documented in RT and RP series.
The reported 10−year incidence of PSA relapse is extremely variable
and ranges from a low of 15% to a high of over 60% (2, 5−12). The well
known factors adversely affecting prognosis, among others, include
tumor stage, Gleason's score, pre−treatment PSA, radiation dose
and technique, PSA nadir following RT and its timing, PSA doubling
time, etc. The unfortunate aspect of many published reports is a
paucity of data on these important risk factors to allow the reader to
understand the outcome data. This is also true for many reports of
RP series. An additional problem is represented by multiple definitions
of PSA failure making interpretation of reported data difficult.
In January 2005, the Second Consensus Conference of Radiation
Therapy Oncology Group (RTOG) and American Society of
Therapeutic Radiology and Oncology (ASTRO) revised the prior
ASTRO definition of post RT PSA failure (13). The following recommendations
were made: 1. a rise by 2ng/ml or more above PSA
nadir defines PSA failure 2. the date of failure is the date of a report
rather than to backdate. A strict adherence to the above definitions
was strongly recommended by the Consensus Conference panel of
experts.
The appearance of PSA failure in RT treated patients is a major
event. It may lead to a local recurrence causing all well known management
problems resulting in a sharp decrease in quality of life.
Local recurrence is initially asymptomatic with subsequent pelvic
and systemic symptoms and signs of recurrence being common.
About 50% of patients with clinical recurrence die within 10 years.
PSA failure predicts well (75%) positive prostatic biopsy and predicts
in 50% of patients clinical recurrence within 1 to 4 years. On the
other hand, a not elevated PSA is associated with < 5% incidence of
clinical recurrence. Additionally, PSA failure may lead to development
of metastasis leading to patient death. Prior to any management
decision following a diagnosis of PSA failure, other relevant
factors need to be carefully considered. These include, among others:
clinical and pathological stage, pre−RT PSA and its velocity, the
duration from completion of RT to PSA relapse (with those >2 years
from RT having a better prognosis), PSA nadir (nPSA), and PSA
doubling time (PSA−DT). Patients with PSA−DT>10 months were
well documented to have a better prognosis than those with a shorter
PSA−DT. Patients with Gleason's score GS>7 have a poor prognosis
compared with those of GS 5−6. The importance of nPSA was
well investigated in a multi−institutional study of 4,839 RT treated
patients between 1986 and 1995 (12). In a multivariate analysis,
times to nPSA and nPSA were found to be predictive of PSA failure
and clinical failure as well as predictive of metastatic disease, which
was true in all risk categories. Higher RT doses were predictive of
lower nPSA, longer time to nPSA and improved incidence of PSA
free survival.
Salvage therapy
Once a diagnosis of biochemical failure has been made and multiple
risk factors carefully evaluated, a search to identify likely site(s) of
possible clinical recurrence can be initiated. Patients with early postradiation
PSA failure (within 1 year), rapid PSA−DT (<10 months)
have >50% probability of developing metastatic disease at 5 years
(14, 15). Detailed history and physical examination are frequently
very helpful in making a diagnosis of a local and/or distant recurrence.
Careful assessment of risk factors and the use of appropriate
imaging to include TRUS can also help to identify the site of local
or distant recurrence. If a local (pelvic) recurrence is suspected
based on the above measures, a TRUS or computerized tomography
assisted needle biopsy may histologically confirm a recurrence and
allow for further histological studies to identify important risk factors.
Evaluation of any therapeutic intervention has to be based on numerous
patient and tumor related factors. It is of major importance
to consider the treatment of a patient with PSA relapse as opposed
to a treatment program designed for an elevated PSA in the absence
of other considerations. An additional important and relevant factor
in this complex process includes the lack of effective curative therapy
in the overwhelming (90%) majority of patients (16, 17). Patient
must be informed in detail on all of the available treatment options,
including watchful waiting. The likely treatment outcome needs to be
presented as well as a likely impact of the proposed treatment on the
patient's quality of life. Prior to an application of potentially curative
therapy, major effort needs to be made to precisely define local
recurrence and exclude a possibility of metastatic disease. It is of relevance
at this time to quote Dr. Willet F. Whitmore, Jr. of Memorial
Sloan−Kettering Cancer Center in New York who made the following
statements more than 20 years ago: „Is cure possible? Is cure
necessary? Is cure possible only when it is necessary?” (16). It is believed
that we should be guided in our decision making process by
the above important and relevant statements.
Surgery
Surgery remains the most effective salvage therapy for patients with
radio recurrent CaP. Surgical treatment options include salvage RP,
laparoscopic prostatectomy and cryosurgery.
Salvage RP
The aim of the salvage procedure, consisting of RP or cystoprostatectomy,
is a total resection of entire known tumor in the pelvis.
Salvage RP has been reported infrequently since the late 1970's
when Willet Whitmore reported treatment outcomes following salvage
RP (18). Treatment outcomes in that report were not very good
and the incidence of surgical complications was high. Following this
report, urologists were reluctant to perform salvage RP due to a well
known problem of defining a local recurrence and fear of severe
treatment toxicity (19). In the past 15 years, there has been an
increase in the number of published reports on this subject. There
was a sharp decrease, reported by most investigators from large university
centers in the incidence of surgical complications and mortality.
Appropriate patient selection and experience of a surgeon represent
a key to a successful outcome following a salvage procedure.
Patients selected for salvage of post−radiation failure are expected to
meet the following criteria: 1. confirmation of local recurrence by
biopsy 2. no seminal vesicle involvement 3. no positive pelvic lymph
nodes 4. no metastatic disease 5. patient's good performance status
with a projected survival = 10 years 6. treatment should be undertaken
in a major urologic center with appropriate surgical expertise.
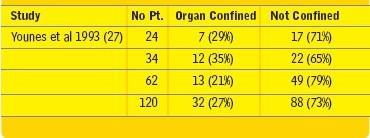
In the three published studies from Mayo Clinic, University of Southern
California (USC) and Baylor University consisting of a total of 120
patients, a sharp decrease in the incidence of surgical complications
and mortality was demonstrated (19). The problem, however, was a
low (27%) incidence of histologically confirmed organ confined disease
(Table 2). The presence of a recurrent tumor outside of the
prostate makes a cure following the salvage procedure unlikely.
|
Pathological finding after salvage RP (19) |
 |
A recent USC report is of interest to consider (16). Between 1983
and 2002, in a total of 2,739 patients, RP was performed including
51 (1.9%) salvage RP's for a biopsy confirmed post−radiotherapy
failure. Median follow−up from a completion of RT to salvage RP
was 5.2 years and median follow−up from the salvage procedure was
7.2 years. No hormonal therapy was given in this group of 51
patients. The 5−year progression−free survival (PFS) was 47% and
median PFS survival was 4.8 years. There was a strong correlation
between pathological stage and PFS. It was 100% for pT2, 35% for
pT3 and 0% for pTxN(+), p<0.001. PSA = 5 ng/ml was predictive
of organ confined disease and PFS, p< 0.001. There was no post
surgical mortality with the incidence of important surgical complications
of <3%. Bladder neck contracture was noted in 41% within
a median of 10 months of surgery. Of the 21 patients so affected,
only 1 was not successfully treated for this complication. Overall,
there was an excellent quality of life outcome with a frequent use of
penile prosthesis and artificial sphincter inserted during the salvage
procedure. Similar excellent outcomes of salvage RP were reported
by other investigators (15, 17, 19, 20−22).
Salvage Laparoscopic Prostatectomy
Laparoscopic surgery appears as an interesting salvage treatment
modality in the management of patients with post−radiotherapy PSA
failure. Very little published data is available for a review. The only
reported study consisted of 7 patients treated in Institut Montsouris
of Universite Pierre et Marie Curie in Paris, France (23). The procedure
was reported to be safe and feasible. Treatment outcome is difficult
to evaluate due to a very limited follow−up.
Cryosurgery
Multiple studies have been reported on the use of cryosurgery in
patients presenting with PSA failure following radiotherapy (19). In
three published studies on 140 patients the treatment tolerance was
good. A single mortality was noted. Longer follow−up is required for
the evaluation of treatment results.
Brachytherapy
Salvage brachytherapy in patients with PSA failure following external
beam radiotherapy appears to have relatively limited usefulness.
An interesting study on salvage brachytherapy was recently reported
from Mayo Clinic (24). A total of 17 biopsy confirmed patients with
post radiotherapy local recurrence and a median PSA of 4.7 ng/ml
received a 3−month course of androgen deprivation therapy followed
by ultrasound guided brachytherapy. A median follow−up was 44
months and the actuarial 4−year biochemical control rate was 75%.
Significant GU (Grade 3+4) toxicity was reported in 8 (47%)
patients, while 6 (35%) had Grade 2+3 GI toxicity. It is apparent
that salvage brachytherapy needs further refinement in order to
reduce the incidence of treatment toxicity.
Deep hyperthermia−RT combination
Highly selected patients with proven local recurrence following a
course of external beam irradiation may be considered for deep
regional hyperthermia in combination with a short (about 40 Gy)
course of focused external beam radiotherapy. A report of Phase I
study from USC on the treatment of 20 locally advanced patients
with local recurrence following definitive external beam radiotherapy
is of interest (25). These 20 patients were defined as end−stage
loco−regional disease not suitable for other forms of salvage therapy.
Complete response was obtained in 4 (20%), and partial response in
4 (20%) of these patients. A total of 15 (75%) patients experienced
major and long−lasting improvement in the quality of life. The 3− and
5−year actuarial survival was 82%. This treatment combination
needs to be re−evaluated in view of major technological improvements
of hyperthermia techniques and instrumentation.
Palliative therapy
An overwhelming (about 90%) majority of patients, who demonstrate
PSA failure following definitive external beam irradiation, are
not suitable candidates to be considered for potentially curative local
therapy. The most effective therapy for patients with recurrent
advanced loco−regional or metastatic disease is hormonal therapy
(HT) (26). The problem is that virtually all patients receiving HT
sooner or later become refractory to this treatment. Additionally,
patients show frequent treatment toxicity which sharply reduces their
quality of life. The issue of the timing of initiating HT in patients
with PSA failure remains controversial. The treatment decision
needs to be carefully considered and its impact clearly discussed
with patients. It is of particular relevance and importance in a majority
of PSA relapse patients who remain asymptomatic sometimes for
a very long period of time. A therapeutic intervention, which is palliative
in nature in this large group of patients, is rather difficult. At
USC, in those who are not suitable candidates for curative therapy,
we discuss in great detail with each patient his PSA increase and its
implications on longevity and the quality of life. Patients remain in
our follow−up clinic for an indefinite period of time and are seen as
frequently as necessary. HT is typically recommended if PSA exceeds
20 ng/ml, there is a rapid PSA−DT or patient becomes symptomatic
due to his progressive cancer.
Summary
It is apparent that efficacy of salvage RP has substantially improved
over the past two decades. The incidence of important treatment toxicity
is low and mortality is much less than 1%. There are two important
remaining problems in salvage RP. First relates to a very small
(<5%) number of patients who can be accepted for this salvage therapy.
Second problem to a limited patient access, due to the need for
the treatment to be given in a major properly equipped and staffed
medical center. Laparoscopic prostatectomy, cryosurgery and
brachytherapy represent emerging salvage therapies, which require
more studies to establish their indications in patients with PSA
relapse following definitive RT. Major research efforts need to be
directed towards the establishment of effective therapy for the 90%
of patients who at the present time do not qualify for curative therapy.
